Sialanar® (glycopyrronium bromide) is licensed for the symptomatic treatment of severe sialorrhoea in children and adolescents aged 3 years and older with chronic neurological disorders1
Discover the topline results on this page or download the publication below.

Study Design2
Study Design
- A double-blind, randomised, clinical phase IV trial comparing Sialanar® with placebo, in addition to continued oral rehabilitation.
- 87 children (3-17 years) with neurodisabilities and severe sialorrhoea (modified Teachers Drooling Scale ≥6) were randomised 1:1 to Sialanar® or placebo, in addition to non-pharmacological standard care during a 3-month double-blinded period (participants are then invited into a 6-month, open-label study extension period).
- The dose was titrated over a period of up to 4 weeks.
- Primary endpoint: the change from baseline to day 84 in the Drooling Impact Scale (DIS) score.
- Secondary endpoints: efficacy,* safety and QoL including responders (DIS improvement ≥ 13.6 points), good responders (DIS improvement ≥ 28 points) and overall changes in DIS - endpoints were assessed at varying time points respectively.
*The efficacy endpoints were assessed in a hierarchal sequence.
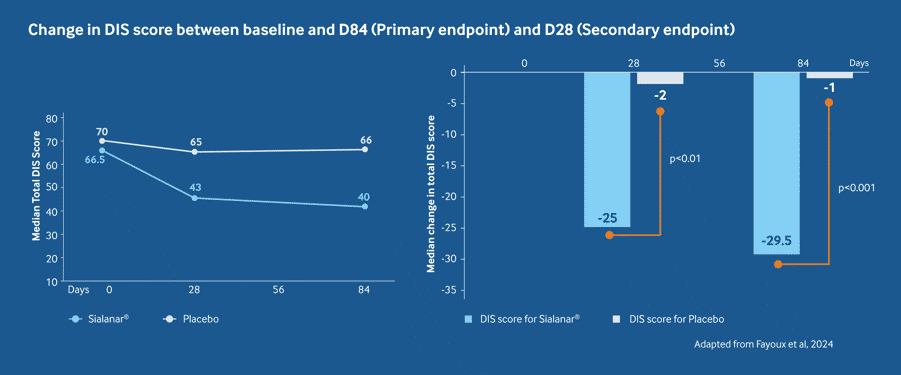
Efficacy - Sialanar® significantly improves drooling vs placebo2
Sialanar® provides significantly greater Drooling Impact Score improvements vs placebo at day 84 (median: -29.5 vs -1; p<0.001)2
Sialanar® significantly reduced bibs/clothing usage vs placebo2
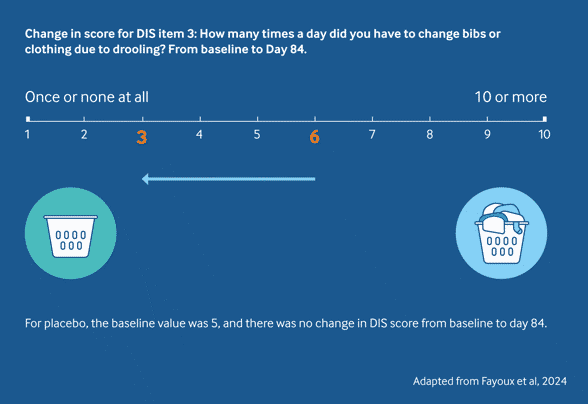
A significant reduction in the median number of bibs/clothes used per day was seen with Sialanar® (-3) compared to placebo (0) from baseline to day 84, helping to reduce costs and enabling more time for carers to focus on other areas of care.2
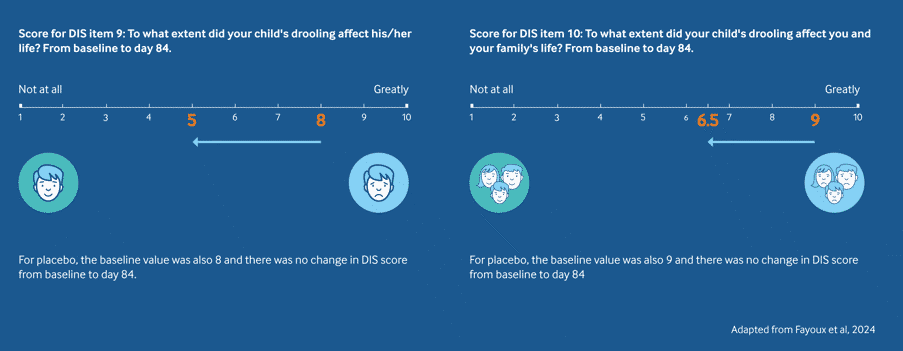
QoL - Sialanar® significantly reduces the impact of drooling on quality of life vs placebo2
Sialanar® is effective at reducing the impact of drooling on the child's and family's day-to-day life vs placebo2
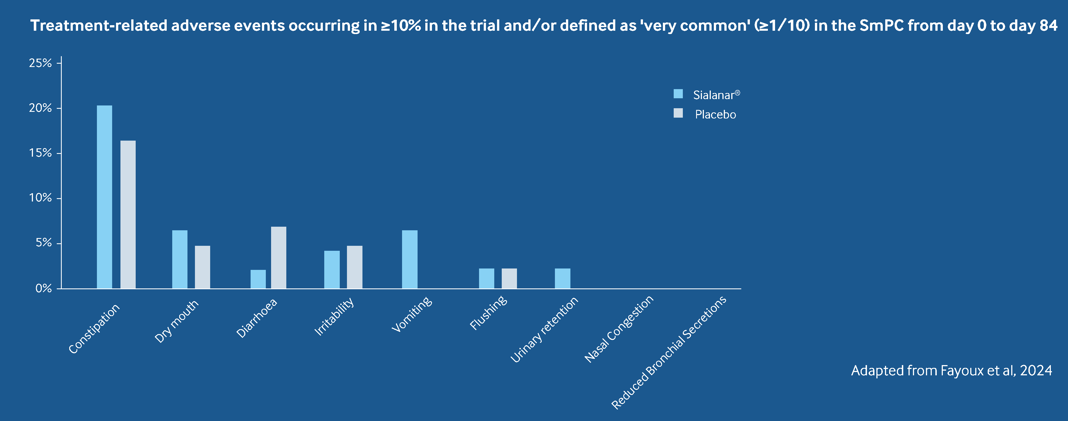
Tolerability - Sialanar® is well tolerated2
The most common treatment-related adverse event in both treatment arms was constipation (20.5% for Sialanar vs 16.3% for placebo)2
Submit the form to arrange a discussion on the SALIVA trial with the Proveca team.











