Tailored titration for flexible and accurate dosing1
It has raspberry flavouring, a titration schedule based upon patient’s weight and is suitable for administration orally and via feeding tubes.1
60% less volume vs.1mg/5ml glycopyrronium oral solutions, helping to improve patient acceptability and tolerability1-5
Minimal excipients to reduce toxicity risk - sugar, alcohol and sorbitol free 1,7,8
Why is volume important?
- The dose volume is a major consideration for the acceptability of a liquid formulation6
- The maximum recommended single dosing volume for paediatric liquid formulations for typically developing children is <5ml for children under 5 years and <10ml for children of 5 years and older5
- Initial research suggests even Cerebral Palsy patients at EDACs level I may have a reduced dysphagia limit in comparison to typically developing children, with a median of 7ml.10
- Eating, drinking and swallowing difficulties (dysphagia) are common in children with neurodisability6
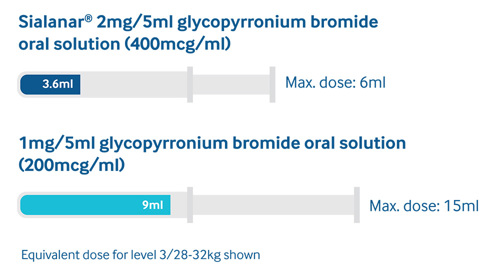
Sialanar® is designed for children and has the lowest volumes for equivalent doses of any licensed glycopyrronium bromide oral solution.1-4
Why are excipients important?
- Excipients are a necessary component of almost all medicines, but they may be present in quantities which are potentially harmful to babies and children6
- Excipients used in adult formulations may not be appropriate for paediatric use so have the potential to lead to adverse effects8
- Oral doses of sorbitol which are greater than 140mg/kg/day may result in increased gastrointestinal (GI) side effects, including osmotic diarrhoea, abdominal pain, bloating and GI discomfort7,8
Watch the excipients animation
Sialanar® is designed for children, using sucralose as a sweetener instead of sorbitol2,3,4 and is suitable for patients on a ketogenic diet (<5mg/ml carbohydrate)1,11
Why is treating drooling important?
Sialorrhoea can cause significant clinical problems and negatively impact quality of life for patients and carers12,13
Physical consequences
-
Posterior sialorrhoea: can lead to aspiration, increasing the risk of respiratory infections, hospitalisations and mortality14-16
- Anterior sialorrhoea: can cause infections, macerated skin, interference with feeding and dehydration13
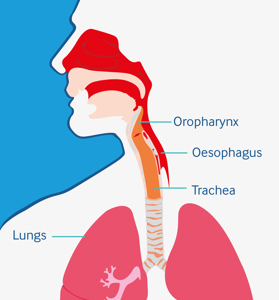
- Aspiration can develop silently so may not be diagnosed prior to development of more serious lung issues14
- Respiratory illness is the leading cause of hospitalisations and mortality in young people with cerebral palsy, 25% of patients (4-5 GMFCS) suffer from chronic respiratory problems16,17
Management of sialorrhoea is recommended to reduce risk factors for aspiration, helping to prevent further respiratory illness15
Psychosocial concerns
- Social embarrassment, isolation and low self-esteem
- Increased dependency and level of care
- Barriers to education (damage and inability to share books / computers)
Dosing and Titration
Clinical Evidence
Glycopyrronium has demonstrated lower rates of side effects18 and treatment cessation18,19 vs. hyoscine.